3.8. Hoàn thành các phương trình hoá học sau :
(1) \(C{O_2} + Mg\)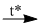
(2) \(C{O_2} + CaO \to \)
(3) \(C{O_{2(dư)}} + Ba{(OH)_2} \to \)
(4) \(C{O_2} + {H_2}O \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} \)
(5) \(C{O_2} + CaC{O_3} + {H_2}O \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} \)
(6) \(C{O_2} + {H_2}O\)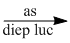 \({C_6}{H_{12}}{O_6}\) + ?
\({C_6}{H_{12}}{O_6}\) + ?
Advertisements (Quảng cáo)

(1) \(C{O_2} + Mg\)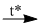 \(2MgO + C\)
\(2MgO + C\)
(2) \(C{O_2} + CaO \to \) \(CaC{O_3}\)
(3) \(C{O_{2(dư)}} + Ba{(OH)_2} \to \) \(Ba{(HC{O_3})_2}\)
(4) \(C{O_2} + {H_2}O \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} \) \({H_2}C{O_3}\)
(5) \(C{O_2} + CaC{O_3} + {H_2}O \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over{\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} \) \(Ca{(HC{O_3})_2}\)
(6) \(C{O_2} + {H_2}O\)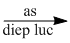 \({C_6}{H_{12}}{O_6}\) + \(6{O_2}\)
\({C_6}{H_{12}}{O_6}\) + \(6{O_2}\)
