9.36. Viết phương trình hoá học thưc hiện các biến hoá dưới đây (mỗi mũi tên là một phản ứng) :
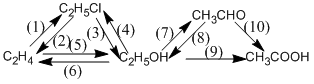

(1) \(C{H_2} = C{H_2} + HCl\)  \(C{H_3} - C{H_2} - Cl\)
\(C{H_3} - C{H_2} - Cl\)
(2) \({C_2}{H_5}Cl + NaOH\)  \(C{H_2} = C{H_2} + NaCl + {H_2}O\)
\(C{H_2} = C{H_2} + NaCl + {H_2}O\)
(3) \({C_2}{H_5}Cl + NaOH\) 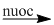 \({C_2}{H_5}OH + NaCl\)
\({C_2}{H_5}OH + NaCl\)
(4) \({C_2}{H_5}OH + HCl\) 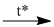 \({C_2}{H_5}Cl + {H_2}O\)
\({C_2}{H_5}Cl + {H_2}O\)
(5) \({C_2}{H_4} + {H_2}O\) 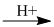 \({C_2}{H_5}OH\)
\({C_2}{H_5}OH\)
(6) \({C_2}{H_5}OH\) 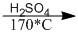 \({C_2}{H_4} + {H_2}O\)
\({C_2}{H_4} + {H_2}O\)
Advertisements (Quảng cáo)
(7) \({C_2}{H_5}OH + CuO\) 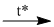 \(C{H_3}CHO + Cu + {H_2}O\)
\(C{H_3}CHO + Cu + {H_2}O\)
(8) \(C{H_3}CHO + {H_2}\) 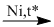 \({C_2}{H_5}OH\)
\({C_2}{H_5}OH\)
(9) \({C_2}{H_5}OH + {O_2}\) 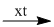 \(C{H_3}COOH + {H_2}O\)
\(C{H_3}COOH + {H_2}O\)
(10) 2\(C{H_3}CHO + {H_2}\) 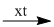 2\(C{H_3}COOH\)
2\(C{H_3}COOH\)
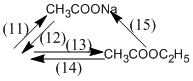
(11) \(C{H_3}COOH + NaOH \to \) \(C{H_3}COONa + {H_2}O\)
(12) \(C{H_3}COONa + {H_2}S{O_4}\) \( \to C{H_3}COOH + N{a_2}S{O_4}\)
(13) \(C{H_3}COOH + {C_2}{H_5}OH\) 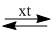 \(C{H_3}COO{C_2}{H_5} + {H_2}O\)
\(C{H_3}COO{C_2}{H_5} + {H_2}O\)
(14) \(C{H_3}COO{C_2}{H_5} + {H_2}O\) 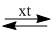 \(C{H_3}COOH + {C_2}{H_5}OH\)
\(C{H_3}COOH + {C_2}{H_5}OH\)
(15) \(C{H_3}COO{C_2}{H_5} + NaOH\) 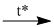 \(C{H_3}COONa + {C_2}{H_5}OH\)
\(C{H_3}COONa + {C_2}{H_5}OH\)
