2.19. Hoàn thành các phương trình hoá học sau đây :
1. \(F{\rm{e}} + HN{O_{3(dac)}}\) 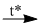 \(N{O_2} \uparrow \) + ? + ?
\(N{O_2} \uparrow \) + ? + ?
2. \(F{\rm{e}} + HN{O_{3(loang)}} \to NO \uparrow \) + ? + ?
3. \(F{\rm{eO}} + HN{O_{3(loang)}} \to NO \uparrow \) + ? + ?
4. \(F{{\rm{e}}_2}{{\rm{O}}_3} + HN{O_{3(loang)}} \to \) ? + ?
5. \(FeS + {H^ + } + N{O_3}^ - \to {N_2}O \uparrow \) + ? + ? + ?
Advertisements (Quảng cáo)

1. \(F{\rm{e}} + 6HN{O_{3(dac)}}\) 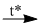 \(3N{O_2} \uparrow \) + \(F{\rm{e}}{(N{O_3})_3} + 3{H_2}O\)
\(3N{O_2} \uparrow \) + \(F{\rm{e}}{(N{O_3})_3} + 3{H_2}O\)
2. \(F{\rm{e}} + 4HN{O_{3(loang)}} \to NO \uparrow \) + \(F{\rm{e}}{(N{O_3})_3} + 2{H_2}O\)
3. \(3F{\rm{eO}} + 10HN{O_{3(loang)}} \to NO \uparrow \) + \(3F{\rm{e}}{(N{O_3})_3} + 5{H_2}O\)
4. \(F{{\rm{e}}_2}{{\rm{O}}_3} + 6HN{O_{3(loang)}} \to \) \(2F{\rm{e}}{(N{O_3})_3} + 3{H_2}O\)
5. \(8FeS + 26{H^ + } + 18N{O_3}^ - \to 9{N_2}O \uparrow \) + \(8F{{\rm{e}}^{3 + }} + 8S{O_4}^{2 - } + 13{H_2}O\)
