Hãy viết ba sơ đồ điều chế cao su butađien đi từ ba loại nguyên liệu khác nhau có sẵn trong thiên nhiên.
Đáp án
*) Đi từ dầu mỏ:
Dầu mỏ \(\eqalign{ & \buildrel {Crackinh} \over\longrightarrow {C_4}{H_{10}}\buildrel {Crackinh} \over\longrightarrow {C_2}{H_6}\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{askt}^{ + C{l_2}}} {C_2}{H_5}Cl \to \cr & \cr} \)
\(\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{t^o}}^{ + NaOH\; loãng}} {C_2}{H_5}OH\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{t^o}}^{xt}} {C_4}{H_6}\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{xt,{t^o},p}^\text{Trùng hợp}} \) 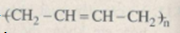
*) Đi từ than đá, đá vôi
\(\eqalign{ & CaC{O_3}\buildrel {{{900}^o}C} \over \longrightarrow CaO\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{{2000}^o}C}^{ + C}} Ca{C_2}\buildrel { + {H_2}O} \over \longrightarrow {C_2}{H_2} \to \cr & \mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{{60}^o} - {{80}^o}C}^{ + {H_2}O;HgS{O_4}}} C{H_3}CHO\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{Ni}^{ + {H_2}}} {C_2}{H_5}OH\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{t^o}}^{xt}} {C_4}{H_6} \to \cr} \)
Advertisements (Quảng cáo)
\(\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{xt,{t^o},p}^\text{Trùng hợp}} \) 
*) Đi từ tinh bột, xenlulozơ:
\({\left( {{C_6}{H_{10}}{O_5}} \right)_n}\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{H^ + }}^{ + {H_2}O}} {C_6}{H_{12}}{O_6}\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{{30}^o} - {{35}^o}C}^{enzim}} {C_2}{H_5}OH\)
\(\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{{t^o}}^{xt}} {C_4}{H_6} \)\(\mathrel{\mathop{\kern0pt\longrightarrow}\limits_{xt,{t^o},p}^\text{Trùng hợp}} \) 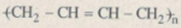
Baitapsgk.com
