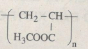Viết phương trình hoá học để hoàn thành dãy biến hoá sau:
a) \(C{H_3}COOH \to C{H_3}COCl \to C{H_3}COO{C_6}{H_5} \)
\(\to C{H_3}COONa \to C{H_4}\)
b)
\(\eqalign{ & C{H_3}C{H_2}COOH \to C{H_3}CHBrCOOH\cr& \to C{H_2} = CHCOOK \to C{H_2} = CHCOOH\cr& \to C{H_2} = CHCOOC{H_3} \to polim e \cr} \)
Đáp án
\(\eqalign{ & a)3C{H_3}COOH + POC{l_3}\buildrel {{t^o}} \over \longrightarrow 3C{H_3}COCl \cr&+ {H_3}PO4 \cr & C{H_3}COCl + {C_6}{H_5}OH\buildrel {{t^o}} \over \longrightarrow C{H_3}COO{C_6}{H_5} \cr&+ HCl \cr & C{H_3}COO{C_6}{H_5} + 2NaOH\buildrel {{t^o}} \over \longrightarrow C{H_3}COONa \cr&+ {C_6}{H_5}Na + {H_2}O \cr & C{H_3}COONa + NaOH\buildrel {CaO,{t^o}} \over \longrightarrow C{H_4} \uparrow + N{a_2}C{O_3} \cr & b)C{H_3}C{H_2}COOH + B{r_2}\buildrel {{t^o},Pđỏ} \over \longrightarrow C{H_3}CHBrCOOH \cr&+ HBr \cr & C{H_3}CHBrCOOH + 2KOH\buildrel {{t^o},{C_2}{H_5}OH} \over \longrightarrow\cr& C{H_2} = CHCOOK + KBr + 2{H_2}O \cr & \cr} \)
Advertisements (Quảng cáo)
\(C{H_2} = CHCOOK + HC{l_{{\rm{dd}}loang}} \to \)
\(C{H_2} = CHCOOH + KCl\)
\(C{H_2} = CHCOOH + C{H_3}OH \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over {\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}}\)
\(C{H_2} = CHCOOC{H_3} + {H_2}O\)
\(nC{H_2} = CHCOOC{H_3}\buildrel {xt,{t^o}} \over \longrightarrow \)